Advanced Trace Metal Monitoring
Modern Water is expert in the design, development and provision of analytical instruments for monitoring trace/ heavy metals in water, soil, food and industrial process streams. Our systems use solid state electrodes to perform voltammetry for the analysis of metals in solution.
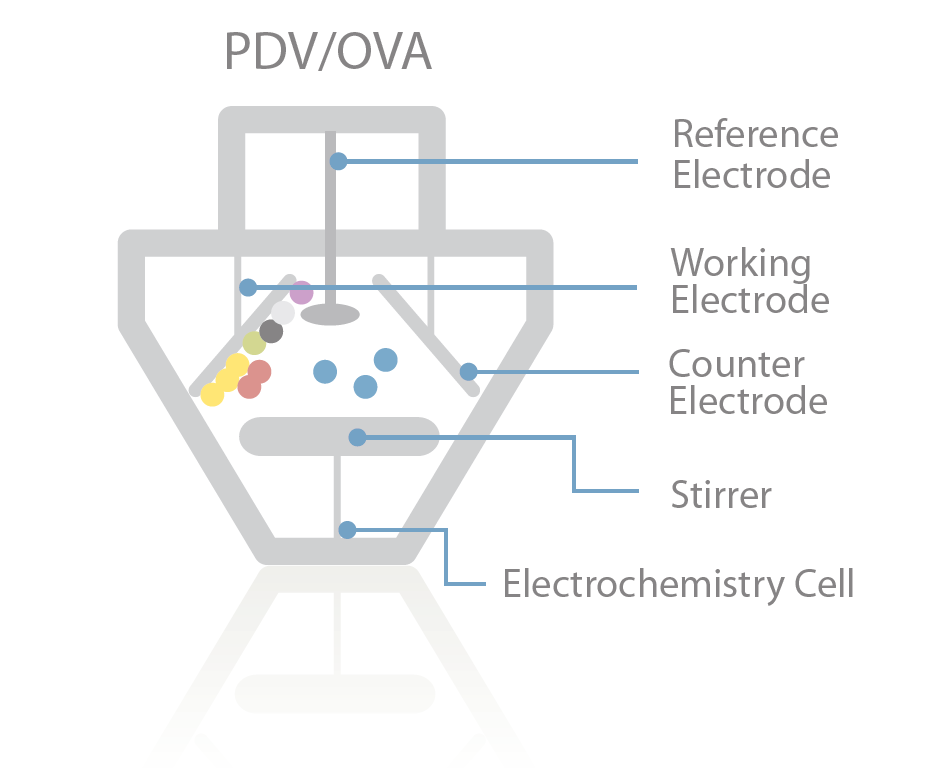
Process Explained
In voltammetry metals are drawn onto the working electrode when a specific voltage is applied to the water sample under test. When a stripping voltage is applied, the metals return to the sample solution, generating a small current. Each metal has a specific voltage at which it returns to solution. So the metal is identified by its stripping voltage, and the current generated is proportional to the concentration of metal in the sample.